In conversation with Praxis SCI Accelerate Cohort Member, Comphya
Novel Therapy to Restore Erectile Function
Interview with Rodrigo Fraga-Silva, co-founder and CEO, Mikael Sturny, co-founder and CTO, and Fiona Joseph, Director of Clinical and Regulatory Affairs
Comphya, a Swiss medical device company, is developing the first implantable neurostimulator to treat erectile dysfunction (ED). The device, CaverSTIM, aims to replace current treatments such as intrapenile injections and penile implants that many patients with spinal cord injury (SCI) avoid. This is often due to the severe side effects, such as pain, discomfort, bleeding, hematoma, priapism, and penile fibrosis. A penile prosthesis is also the last therapeutic option as it is an irreversible.
CaverSTIM offers a safer, non-traumatic, comfortable, spontaneous and more effective alternative to traditional treatments. The technology implants electrodes in the pelvic cavity to selectively activate and trigger penile erection. With SCI this pathway is often disrupted, which in turn may prevent intercourse and ejaculation. CaverSTIM will provide self-controlled stimulation to achieve and maintain a physiological penile erection.
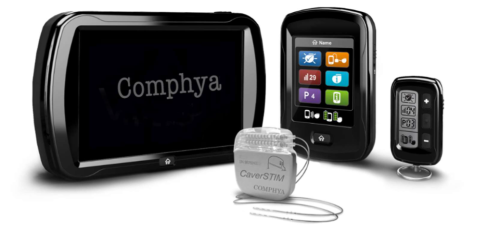
CaverSTIM is an active implantable medical device composed of: I) the implantable pulse generator (IPG) connected to II) an electrode paddle, III) external controllers (physician and patient control units), and IV) wireless recharger.
Comphya emerged from EPFL in Lausanne, Switzerland (École Polytechnique Fédérale de Lausanne), a rich academic environment with existing technology development aimed to improve the quality of life for people with SCI.
What led you (education, experience, people) to a career focused on medical technology and, in particular, medical technology for people living with SCI?
MS: Our three co-founders, Rodrigo Fraga, Nikos Stergiopulos and myself (Mikael) have our roots in basic science and academia. But we realized that more was needed to translate science into real impact and benefit society. The reason why we founded Comphya was to deliver technology and improve the quality of life for people. In my case (MS), I did a PhD in bioengineering, so I was immersed in the field of medical technology. The next step was to found the company, to make it real, and not just research and science.
RD: Indeed, the creation of Comphya will allow our research to reach individuals and benefit their lives. Comphya emerged from EPFL in Lausanne (École Polytechnique Fédérale de Lausanne), a rich academic environment with existing technology development aimed to improve the quality of life for people with SCI.
Team (L-R): Mikael Sturny, CTO and co-founder, Fiona Joseph, Head of Clinical Affairs, Rodrigo Fraga-Silva, CEO, Board member and co-founder, Nikos Stergiopulos, CSO, Board member and co-founder
The development of new technology needs to be focused on the end-user, and we tend to miss it along the journey.
What was your biggest learning/takeaway from the program?
FJ: We have learned how important is to have users’ feedback in the development of a product. We knew it was important from the beginning; the Praxis program provided us with key access to interact with the end-user, and this was really valuable. We learned many aspects on living with SCI that we didn’t know, such as the rehabilitation challenges that patients faced at the hospital and in daily life.
RD: I agree with Fiona. The development of new technology needs to be focused on the end-user, and we tend to miss it along the journey. Praxis promoted an open and frank discussion with people with SCI so our team got consistent feedback to support development.
Do you have a specific example of how that played a role in your learning?
FJ: Yes — it gave us an additional potential application for our device. We learned that triggering a semi erection with lower intensity stimulation could help with [urinary bladder] catheterization. People with SCI have to undergo this procedure several times every day; if our device could trigger a semi erection that could facilitate this catheterization.
MS: We also learned that conception is quite difficult for people living with SCI, and that our technology could potentially also trigger ejaculation; this might be great to help people living with SCI to conceive.
What were the biggest changes to your company’s structure and/or business strategy after or during the program? Are there any shifts you plan on making in your company’s structure and/or business strategy in the next few months?
MS: The program was particularly valuable for us in terms of validating, refining and improving. For example, the feedback really allowed us to validate and refine our reimbursement strategy, confirming the assessment of our consultants on the best strategy possible for North America.
And we had great feedback from the focus group and from the weekly monitoring meetings. They helped us improve the clinical protocols, including the assessment of the new potential benefits, catheterization and ejaculation.
In sum, the general strategy is the same, but it was refined and more features added.
FJ: We also learned an additional reason for payers to reimburse our device. In addition to treating erectile dysfunction, if it helps with catheterization and leads to a decreased number of patients ending in a hospital because of infections, it could be a good reason for payers to reimburse it.

