How Direct Engagement with Users Shaped KeyGrip’s Path Forward
📖 5 min read | #Rehabilitation #MedicalDevices #RegulatoryPathway #HumanCenteredInnovation
How direct engagement with users shaped KeyGrip’s path forward
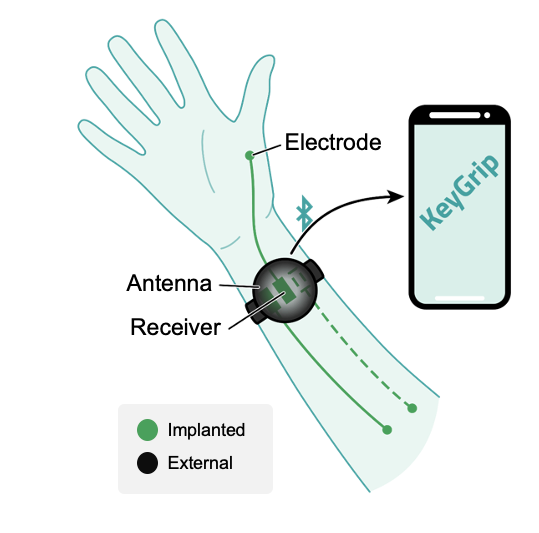
For Megan Moynahan, a biomedical engineer and founder of KeyGrip, the goal was clear—develop an implantable device to restore hand function for people with cervical spinal cord injuries (SCI). The science was strong, and the need was well-documented. But one crucial question remained: How would potential users and surgeons actually perceive it?
Through the Praxis 2024 SCI Incubate Program, KeyGrip gained direct access to Persons with Lived Experience (PLEX) of SCI and regulatory experts. These conversations reshaped their messaging, reintroduced a product feature they had ruled out, and provided a clear roadmap for entering the Canadian market.
Hearing directly from users changed everything
KeyGrip’s initial assumption was that anyone with SCI seeking more independence would want to improve their hand function. But through focus groups with PLEX members, they uncovered a more complex reality—potential users had concerns about surgical risks, recovery time, and the fear of losing existing function.
Rather than just highlighting the benefits, KeyGrip needed to address fears head-on, ensuring their messaging reassured users and provided clear data on the long-term impact.
“We always knew people valued hand function—but hearing their concerns in their own words made us rethink how we communicate our solution.”
Bringing a feature back into the design
One of the most unexpected takeaways was how users viewed different control methods. Initially, KeyGrip had dismissed voice activation, assuming it wasn’t a priority. But user feedback challenged that assumption.
This insight put voice control back on the table, influencing upcoming development proposals.
“People may not want to talk to their hands in public, but I was really surprised that they are open to voice activation at home, making it a viable control method once again.”
A Clearer Regulatory Pathway for Canada
Beyond product and messaging refinements, Praxis helped KeyGrip navigate the Canadian regulatory landscape, an area they hadn’t initially prioritized. Through mentorship and expert connections, they developed a clear plan for clinical trials and approvals.
This new perspective expanded KeyGrip’s market strategy, ensuring they could plan for approvals in multiple regions early on.
Insights that will shape KeyGrip’s next steps
Unlike traditional incubators with a set curriculum, Praxis adapted to what FMRK needed—whether that was regulatory guidance, market validation, or direct feedback from Persons with SCI Lived Experience (PLEX).
The biggest shift for KeyGrip wasn’t just in strategy—it was in how they approached decision-making. Conversations with PLEX members provided real-world perspectives that no market research could replicate, while regulatory guidance gave Megan and her team the clarity they needed to move forward with confidence.
