In conversation with Praxis SCI Incubate cohort member Purus Innovations

Purus Innovations Co-Founder Dr. Dirk Lange talks about how the company started and its innovative technology to prevent UTIs.
What was the deciding moment in starting the company?
Working with colleagues and patients in the urology space at the University of British Columbia, I encountered indwelling device-associated infections as a significant issue. It made an impact. There is a real need for a solution that’s actually working.
In 2008, I met Purus Innovations Co-founder Jayachandran (Jay) Kizhakkedathu during my postdoc in urologic sciences. Jay is a professor in the UBC Department of Pathology and Laboratory Medicine, the Centre for Blood Research, and the School of Biomedical Engineering. There was a collaboration at the university to prevent orthopedic implant infections, and Jay was the chemist developing an antimicrobial coating that needed to be tested in vivo in an animal model. I developed the in vivo model to test it, and showed how effective it was at preventing infection. Jay asked the question, “Why just orthopedic?” and I thought let’s develop it for urologic devices. The relationship took off.
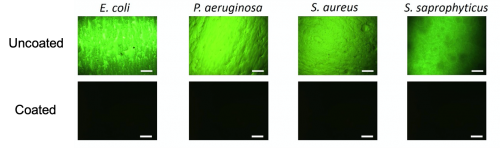
Everyone else was just targeting the bacteria. We developed a coating that has two unique mechanisms working together, that acts to prevent deposition of components and also kills the bacteria resulting in a clean surface.
There are tons of antimicrobial coatings. What sets us apart is how we looked at the research. Why have these past technologies failed? When these coatings were introduced into urine or blood – complex media with proteins – you get the deposition of a conditioning film, a physical barrier on the surface. It renders anything below it ineffective. So how could we address the deposition of the components as well as the bacteria causing infections? Everyone else was just targeting the bacteria. We developed a coating that has two unique mechanisms working together, that acts to prevent deposition of components and also kills the bacteria resulting in a clean surface.
To move the technology forward, it was clear to us that we needed to start a company and hence Purus Innovations was born. Purus is Latin for “clean” or “pure”. In the context of catheters, we’re looking at an antimicrobial technology, but it’s also antibiofouling, meaning it keeps organic and inorganic materials off the surface. Purus covers this thinking.
What led you here in terms of experience and people you met along the way?
We had – and continue to have – invaluable mentorship from key serial entrepreneurs, including Gabe Kalmar and Doug Buchanan through entrpreneurship@UBC. It was an eye-opener. Gabe and Doug got us to think about the business side, how to structure a company and IP development. As inventors we think we’ve got the greatest thing, but they introduced the idea of market research, asking questions with customers. Expert mentors have gotten us to this point.
How are you providing a solution to your customer’s needs?
Working with the Praxis SCI Incubate Program, we are focusing on people living with spinal cord injury (SCI). Individuals with SCI rely on catheters every day to empty their bladders. The introduction of bacteria into the urinary system during this process is one of the most common complications and fears faced by the SCI population. This results in a significant negative impact on their quality of life. Because of this, there is a significant need for technologies that address this issue, lowers the risk for infection associated with indwelling catheters, and lowers the use of antibiotics.

Example of a clinically used urinary catheter coated on the inside and outside surface.