In conversation with Praxis SCI Incubate cohort member Starling Medical

Starling Medical CEO Dr. Alex Arevalos and Director of Product Development Sylvie Kalikoff, MBE, on revolutionizing neurogenic bladder management with an AI and tech-enabled platform.
What was the deciding moment in starting the company?
Sylvie Kalikoff: Starling Medical began as a research project at Rice University in Houston, Texas between myself and the two other founding engineers, Hannah McKenney and Drew Hendricks.
We were part of the same small cohort in the Bioengineering Master’s program, which links engineers with clinicians. Our collaborators at Texas Children’s Hospital across the street brought our attention to the fact that individuals with neurogenic bladder dysfunction, including those living with a spinal cord injury (SCI), need a better way to empty their bladders on a day-to-day basis. Neurogenic bladder is the name given to a number of urinary conditions in people who lack bladder control due to a brain, spinal cord or nerve problem. Currently, patients use catheters five to eight times a day. This leads to a high rate of severe urinary tract infections, or UTIs, that require hospitalization.
Compared to the general population, patients with severe neurogenic bladder are 51X more likely to be admitted to a hospital for a UTI, and the mortality rate resulting from the bacteria overreaction is too high.
Many people think the most significant issue facing those with an SCI is lack of mobility since that is the most visible thing. They don’t think about the biological functions they take for granted, like the ability to pee. The mortality rate of UTIs in the SCI population is as high as 10 to 15 percent.
The problem statement instantly motivated me. My younger sister, Natalie, is severely disabled by cerebral palsy and was born with a neurogenic bladder. She has dealt with the current standard of care methods and catheterization her entire life.
The Starling Medical team and prototype.
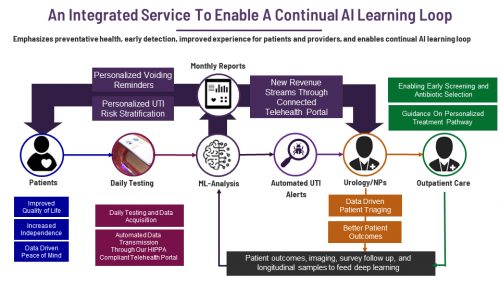
As Hannah, Drew and myself looked into this further, we realized not only was there a large, widespread, and largely underserved clinical need affecting millions worldwide, but there was a significant value proposition. By harnessing artificial intelligence to better diagnose UTIs, we could lower hospitalization rates and decrease costs while increasing the quality of life of these patients and even save lives.
At the individual level, a device like this would be transformative for someone like my sister Natalie. In her case, she can’t walk or talk, but she likes to be out in the world and around people.
Tell us about your customers and potential customers?
Alex Arevalos: There are over 30 million patients in the US – let alone the rest of the world – who suffer from neurogenic bladders. This includes people who had a stroke, have a disease like Parkinson’s, Alzheimer’s, dementia, or multiple sclerosis, have neural tube defects, or are living with a spinal cord injury.
As Sylvie mentioned, the current standard of care is not ideal. Patients must undergo clean intermittent catheterization to drain their bladders many times a day. They also need to take medication to relax their constantly overactive muscles. The catheterization can be painful, time consuming and expensive. It’s also does not fully do the job. Many patients still have leakages, despite being on a regular schedule.
Yet the biggest challenge is catheterization increases the risk of a UTI. Compared to the general population, patients with severe neurogenic bladder are 51 times more likely to be admitted to a hospital for a UTI, and the mortality rate resulting from the bacteria overreaction is too high. These patients aren’t able to feel traditional early signs of a UTI, such as a persistent urge to urinate or a burning sensation when urinating. Urologists are left with subjective screening criteria, which can be difficult for patients and caregivers to adequately assess.
Sylvie: At the individual level, a device like this would be transformative for someone like my sister Natalie. In her case, she can’t walk or talk, but she likes to be out in the world and around people. She is 23 years old and would ordinarily attend a special day program. Because she needs to be catheterized so often, she requires special nursing care. If she had a better solution, she wouldn’t be so isolated.
What specific solution is your company providing?
Sylvie: Our UrinControl System is a temporary urinary prosthesis that is anchored once a month into a patient’s urinary sphincter. The prosthesis is wirelessly driven and has an internal pumping mechanism to drain the bladder at the push of a button. The device also has a pressure relief valve to prevent reflux. This holds continence between bathroom visits but automatically releases when the pressure goes above a certain level.
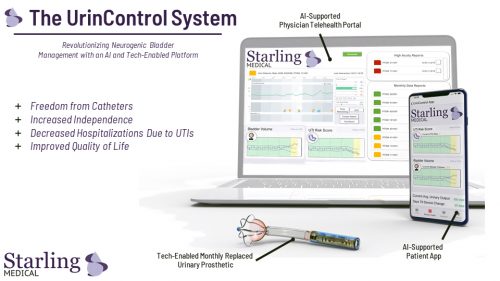
The patient controls the system through their smartphone using the UrinControl app. Information is sent between the prosthesis and their phone with radio frequency powered circuitry. Patients can check the fullness of their bladder, track their urinary output and receive objective, early warnings about the presence of a UTI. They can also lower the bacterial load in the catheter through UV-based active sterilization.