In conversation with Praxis SCI Incubate Cohort Member, Neuro Vigor

CEO & Co-founder Mark Van Fleet talks to us about Tackling Neuropathic Pain with Neuro Vigor
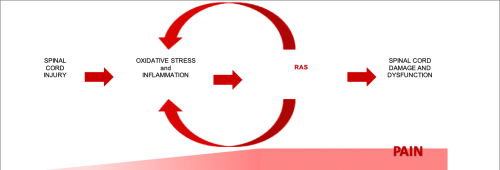
Neuro Vigor is a company based in Indiana developing pharmaceutical therapies to curb inflammation, pain and neuro degeneration. Their first priority is to develop an effective treatment for neuropathic pain for people living with spinal cord injury (SCI), a condition for which current remedies are inadequate. The company was co-founded by entrepreneur Mark Van Fleet who helped spin out research from Purdue University neuroscientist, Dr. Riyi Shi, a pioneer in toxic aldehydes and their reduction. Currently, Neuro Vigor is in the pre-clinical phase of drug development, identifying a lead candidate to lower reactive aldehyde species (RAS), and mitigate or eliminate their role as a major source of neuropathic pain.
RAS are critical contributors to oxidative stress, increase with disease and trauma, trigger inflammation, damage important biomolecules (e.g., DNA, proteins, mitochondria, myelin), and compromise the body’s antioxidant defenses. They have been implicated in the pathology of several medical conditions, including Multiple Sclerosis (MS), SCI, Parkinson’s disease, Alzheimer’s disease, chronic obstructive pulmonary disease (COPD), advanced liver disease, diabetic neuropathy and cardiovascular disease. In addition to work in reducing SCI neuropathic pain through the reduction of RAS levels, Neuro Vigor’s chief scientist, Dr. Riyi Shi, has also demonstrated pre-clinical efficacy of RAS-scavenging compounds in animal models of MS and Parkinson’s disease.
What was your biggest takeaway from the Praxis SCI Incubate Program?
Research conducted by Neuro Vigor prior to entering the program had not fully revealed the impact of neuropathic pain for people living with SCI. Weekly meetings with the Praxis Consumer & Clinical teams, along with focus groups of people with SCI who experience ongoing neuropathic pain, exposed us to the personal consequences of inadequate treatment. This perspective provided invaluable consumer feedback on the need for a more effective treatment. This was further validated by the two clinical panels that Praxis arranged. They confirmed our science and provided feedback on drug development strategy. The panelists, who are leading experts in the field of therapeutic development for chronic pain, will be invaluable connections for our drug development journey and would not have been available without Praxis’ ability to leverage its network.
What were the biggest changes to your company’s structure or business strategy arising out of the program?
The Praxis SCI Incubate program brought about valuable improvements in Neuro Vigor business practices. First, and perhaps the most impactful change , was our team’s ability to communicate the science behind our research more effectively. This has strengthened our grant funding proposals, investment communication, and has already led to new collaboration prospects. Understanding how to communicate complex science and drug development to grant funders, investors and other potential collaborators more effectively is vital to our future success.
We heard from several experts that we will need more time, money, and resources at the discovery phase to be successful. As a result, we are designing a longer drug development runway, budget and timeline that will attract potential partners and investors. We are also recruiting advisors and executives to fill gaps identified during the program.
“The program will stress-test your assumptions about your product and growth strategy against the realities of the market, technology, science, and value to intended consumers. SCI Incubate will uncover new perspectives about your relative strengths and weaknesses, and help you better align your plans with the community you hope to serve and the investors you hope to attract.” Mark Van Fleet, Ceo & Co-Founder

Image of Dr. Riyi Shi
How did the program change how you viewed challenges faced by your customers?
After learning more about how people with SCI are affected by neuropathic pain, we are now integrating consumer perspectives into our planning. This will ensure we address issues earlier in our pre-clinical stage to better prepare for successful clinical trials. Thanks to our focus groups of SCI consumers and clinicians, we now understand that individuals participating in our clinical trials will likely be using a variety of drugs for their neuropathic pain. These trial participants are unlikely to interrupt their current pain treatments in order to enter our trials; our clinical research term will need to balance our priorities with the daily challenges of individuals facing SCI pain. Feedback from clinicians and people with lived SCI experience has informed our decision to conduct trials with participants at the sub-acute stage.
Company Successes Due to the SCI Incubate program:
- Strengthened communication strategies and vehicles, e.g., more focused scientific overviews, website improvements
- Alterations to clinical trial focus and protocols to reflect realities of the SCI community
- Realistic business roadmap sets the company up for successful development and funding
Did your perspective change on how your customers would use your product?
The focus groups set-up by Praxis reinforced the demand for a more effective drug to reduce neuropathic pain. We also have a greater understanding of the importance of oral dosage scheduling and the critical need to control side effects.
What did you get out of the SCI Incubate program?
Interaction with Praxis deepened and broadened our understanding of the impact of neuropathic pain after SCI. We gained a more comprehensive understanding of both the scale and complexity of the need for effective treatment, learning how each individual with SCI has their own unique challenges, and what the true burden of drug side effects are.
The program provided what we were looking for, to grow our connections in the clinical and consumer communities for future collaborations, to attract first-rate advisors, to strengthen our business strategy, and to refine our communication to more clearly explain our science and solution to key audiences.
