In conversation with Praxis SCI Incubate cohort member Spiderwort

Spiderwort CEO & Co-Founder Dr. Charles M. Cuerrier and Business Development Director Dr. Stephen Hanson, MBA, on their cellulose scaffolding that promotes regeneration after SCI.
What led you here in terms of education, experience, and people you met along the way?
Charles M. Cuerrier: I always sensed I would create my own business. I come from an entrepreneurial family. My great-grandparents owned a hotel, my grandparents owned a grocery store, and my father is also an entrepreneur. I was the outsider, pursuing sciences and a doctorate in pharmacology, but the entrepreneurship root is in my genes.
During my postdoc at the University of Ottawa, Andrew Pelling, Daniel Modulevsky and I were working on a new strategy to regenerate tissues using cellulose extracted from plants. We were trying different things to see how structures were organized, and we had good results using cell cultures. Then we started animal studies and saw how well our biomaterial integrated into the body. We knew we had something important on our hands. We showed the results to pathologists – people trained to analyse this type of data – who confirmed we were working on something amazing that could change the regenerative medicine sector and create a new tool in the surgeon’s toolbox.
At that point, I knew I could not let someone else bring this technology to the clinic. I had to lead that process. We obtained a patent with the University of Ottawa and, in 2015, we pushed this innovation into a company called Spiderwort.
Stephen Hanson: My passion for business began while pursuing my PhD in Medical Sciences at McMaster University. I became involved with several businesses at the university, and realized combining science and business as a career made sense. There are a limited number of people that understand both, and I could be a conduit to move innovation from the lab into the market.
I pursued an MBA with a focus on commercializing biotechnology. While completing an internship at the Technology Transfer Office at the University of Ottawa in 2016, I met Charles and learned about Spiderwort’s great potential. I knew I wanted to be part of it and officially joined the team three and a half years ago.
I am proud of the important financing, technology and regulatory milestones we have achieved. The start-up stage for any business is hard work, and Spiderwort is no exception. A large focus has been raising money. In 2018 we had a Friends and Family funding round that raised $500,000 CAD. In October 2019, we closed a Series Seed round of financing with $2.5 million USD. Now we are aiming to raise $15 million. We need that much money because clinical studies cost millions of dollars per trial. Despite the hard work, the start-up stage has also been extremely rewarding and motivating, and we are surrounded by amazing people.
We were trying different things to see how structures were organized, and we had good results using cell cultures. We showed the results to pathologists – people trained to analyse this type of data – who confirmed we were working on something amazing that could change the regenerative medicine sector and create a new tool in the surgeon’s toolbox.
CelluBridge™ provides the scaffolding that works with the body’s own cells to provide a framework for the functional growth and regeneration of neurons.
Tell me about your customers and potential customers?
Charles: Our initial target customers are those classified under the American Spinal Injury Association Impairment Scale as Grade A, which means complete sensory or motor function loss below the level of injury, and we are specifically focusing on their acute phase of treatment. Grade A injuries represent roughly a third of the 19,000 new spinal cord injury (SCI) cases in the United States and Canada each year. Eventually, we hope to expand to those with incomplete injuries – classified as Grade B and C – as well as into the chronic phase of SCI treatment.
Stephen: We are working to bring this solution to customers as soon as possible. Realistically, it will take some time to get there. We are currently undertaking preclinical animal studies. In 2019, we received a “Breakthrough Device Designation” from the FDA, which accelerated our pace to move into clinical trials. That being said, the R&D to develop technology like this is very complex. The biomaterial is long-lasting technology that is actually implanted into patients. We need to have the right results in place to show it is safe.
Our lead product – CelluBridgeTM – is a cellulose scaffold that is implanted in a patient’s spinal cord within 96 hours after injury. This array of microchannels, or small tubes, guide the regenerating neurons to repair the injury.
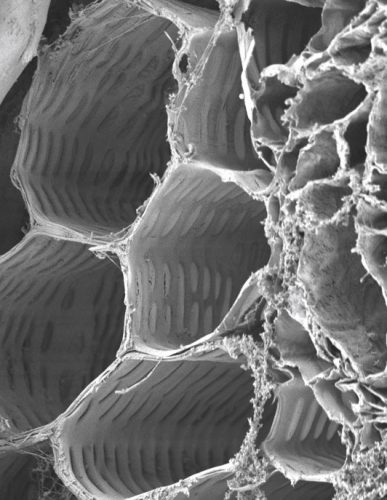
Spiderwort’s spinal cord scaffold implant, CelluBridge™, has been designated by the FDA as a Breakthrough Device.

