This is the breadcrumbs block. Breadcrumb will populate on actual page.
Inaugural SCI Accelerate alum, Rehabtronics Develops Novel Neurostimulation Product to Treat and Prevent Pressure Injuries
Praxis exists to lead global collaboration in spinal cord injury research, innovation and care. We translate knowledge to bridge health evidence with real-world delivery. The word praxis means the practical application of a theory – moving knowledge into action. We measure our success through impact, how Praxis makes a difference to the SCI community.
Rehabtronics
Rehabtronics, a BC-based medical start-up and inaugural Praxis SCI Accelerate program alum, has developed a novel neurostimulation medical device to treat and prevent pressure injuries. A wireless stimulator delivers intermittent electrical stimulation to the underlying tissues, restoring blood flow and tissue oxygenation. Treatment time is only 10 to 15 minutes on the affected area per day. Praxis has supported the novel technology behind Rehabtronics for over 10 years, starting when it was a research project at the University of Alberta’s Neuroscience and Mental Health Institute.
“The quality of mentors, advisors, and researchers at Praxis is world-class: in just a few short weeks, we gained tremendous value.” — Rahul Samant Rehabtronics CEO & Founder
Why?
Most people living with spinal cord injuries (SCI) experience at least one or more pressure injuries (PI) in a lifetime. As part of the daily care plan, this means paying close attention to skin prone to damage. An untreated PI can quickly progress into full thickness damage and systemic infection, greatly impacting quality of life or even causing death. In economic terms, a 2021 Praxis report concluded that pressure ulcers in the Canadian population with SCI cost between $410 and $907.9 million annually.
It’s worth noting though that these stats include only direct costs, mainly from hospital administrative data such as admission costs and does not account for the indirect costs of PIs. Additional costs of PIs for people living with SCI may include lost time from work, impact on families and caregivers, and expenses associated with morbidity and mortality. This further increases the economic burden of PIs and the potential cost savings resulting from interventions that aim to reduce its occurrence and/or severity.
How?
Rehabtronics, which has a strong presence in neurostimulation for rehab, tackles PI management with state-of-the-art neurostimulation tech in its Prelivia medical device. On joining the first cohort of Praxis’s inaugural SCI Accelerate program in April 2020, the company had limited funding, clinical validation, or regulatory approval. The program connected Rehabtronics with a network of entrepreneurial, regulatory, reimbursement and clinical mentors to develop a realistic regulatory plan, helping the team identify weaknesses and develop a roadmap to effective market entry.
During and following the program, Rehabtronics received their Investigational Testing Authorization (ITA) from Health Canada, and within nine months of graduating, gained U.S. Food and Drug Administration 510 (k) Clearance for Prelivia as a Class II medical device. Following these, $1.6 Million oversubscribed seed funding along with $1.25 Million from Genome BC (a grant funder of the Praxis Commercialization Program) funded clinical trials required for Health Canada regulatory approval for sales across North America.
What is Success?
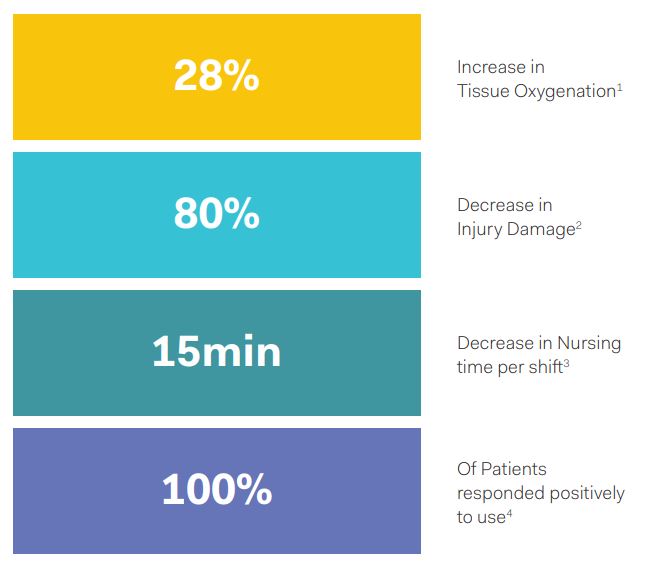
Clinical trial data are very encouraging, with benefits seen in PI tissue damage limitation, nursing time and patient response. According to James Laskin, Researcher in Residence with the Praxis Commercialization Programs, what’s unique about the Prelivia system is that it could be used in both prevention and treatment. It is simple to use, easy to apply, non-invasive, and has minimal impact of functional activities.
“Pressure injuries kill!! Pressure injuries destroy quality of life! Depending on size, location and extent they can keep you in bed for weeks, months, or years. Anything that we can add to our toolkit for prevention and treatment is critically needed and welcomed, by both the SCI community and the healthcare professionals.” — James Laskin, Researcher in Residence, Praxis Commercialization Programs
As a BC-based medical device company, Rehabtronics brings value to a vibrant life sciences ecosystem that is already attracting talent and intellectual property to the province. Not only does having the company and its innovation benefit BC’s economic development, but it also increases access to vital healthcare strategies and technology for residents. As part of the Praxis network, provincial and global, this further supports BC as a hub of spinal cord injury research excellence.